SiliconeImplant.jpg
Photo from Defense Visual Information Distribution Service / Public Domain
The Silicone Implant Problem
Between one and two million women have had silicone breast implants to improve their physical appearance. Within and after thirty years, thousands of women have been questioning their earlier decision, and many seem to be suffering major disabilities as a result of these implants. In 1993 the FDA placed restrictions on the use of silicone for breast implants and currently (1994) there is pending a record $4.7 billion settlement in one of the nation's largest class-action suits resulting from the implant damage.
When the U.S. patent office approved the first implant in 1966, they were not legally required to test out implants, and so long as implants were treated as a "device" rather than a drug, it did not have to pass rigorous testing to satisfy the FDA.
Until 1978 no one outside of the chemical companies had reason to know that the envelope surrounding the implant was permeable to the silicone gel inside. When three researchers reported this fact, the whole world knew why certain people, who had received the implants, were becoming ill.
Silicone had long been thought to be chemically inert. This view has changed in the past 38 years. Not only is the silicone tetramer an immune system stimulant and dysregulator, but also the envelope covering breast implants does not protect the individual from the chemical inside the "sac." Slowly and at different rates the silicone oil leaks through this semi-permeable membrane and is carried around the body by cells of our immune system. "Slow contamination of the body by this chemical and the secondary effects on the body lead to what I am calling the 'Silicone Immune Dysfunction Syndrome'," Dr. Edelson2 says.
There are several forms of the chemical breast implants that can cause a problem. Silicon is the basic element (Si) and probably causes immune system changes. Silica, or SiO2 (chemical formula), is the way it is mined from the earth. Silica is 45% silicon. It's chemical structure is O — Si — O.
Silicone gel, used for breast implants, is a synthetic material containing 38% silicon. The usual implant material is a silicone tetramer (polydimethylsiloxane). There is slow leakage ("bleeding") of the silicone gel from the implants through the semi-permeable membrane envelope and also into and through the capsule that surrounds the implants. This is picked up by the macrophages (scavenger cells) of our immune system and is broken down inside these cells which travel all over the body.
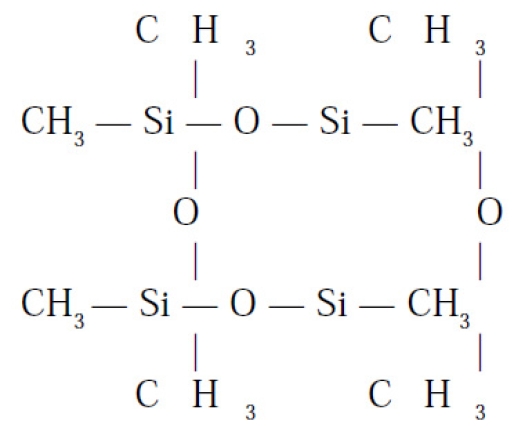
The gel breaks down into Silica (SiO2) and Silicon (Si) which causes an immune system dysregulation. Thus there are antibodies produced against the silicon and also against the silicon and protein complex (organ systems) so that one suffers from an auto-immune illness. Polydimethylsiloxane's chemical structure is shown at right.
There is also damage that is not related to the immune system because the silicone gel causes oxidants (damaging molecules) to be produced that directly damage our cell walls, DNA, and enzyme systems. All of this adds up to slowly developing chronic debilitating illness affecting every organ system of the body.
As expressed by Dr. Edelson,2 and other physicians, the background burden of oxidants and toxins from our surrounding environment, and the food we eat and drink, adds hugely to the already overpowering burden from silicone leakage. Together, silicone and the oxidant burden (bacterial endotxins, food chemicals, and chemicals in our world; i.e., xenobiotics), deplete our natural antioxidant systems. Depletion of our antioxidant systems produces respiratory damage and GI damage, the latter leading to organ damage affecting the immune, respiratory, nervous and endocrine systems, as well as other physical systems. Skin damage is also induced, leading to rapid aging of skin and cancer.
Some women whose implants have ruptured, or who suffer from continual silicone leakage, have had a difficult time finding physicians who will work with them.
According to Stephen Edelson, M.D.,2 Immune Disorder Symptoms, sometimes occurring within months of implant, but often occurring 15 to 20 years after surgical implant, include the following: Peripheral Neuropathy (weakness, tingling, numbness, etc.), Central neurotoxic Neuropathy (cognitive difficulties, memory problems, hyperactivity, attention deficits), Cervical and axillary enlarged or painful lymph nodes, fatigue, malaise, weight gain/weight loss, joint and tendon pain, hair loss, dry eyes & mouth, flu-like symptoms, burning skin, constipation, dizziness, enlarged lymph nodes, depression, thyroid problems, hair loss, night sweats, fibromyalgia (multiple tender areas), Myositis (painful inflamed muscles), abdominal pain, emotional instability, chemical sensitivity, food sensitivity, and Pulmonary Hypersensitivity (shortness of breath).
It is believed that these are only the most observable symptoms. Some patients are only moderately ill, while others suffer damage to more than one organ of the body.
Some people who have Silicone Implant illnesses have been diagnosed by their physicians with Lupus, Raynaud's Sjogren's, Rheumatoid Arthritis, Chronic Fatigue Syndrome and Multiple Sclerosis.
Edelson says that "All of these symptoms are caused by the dysregulation of the immune system and by damage occurring from the free radicals produced by our own system in response to the chemical silicone."2
Before the symptoms begin, there are immune system functional abnormalities that allows physicians to pinpoint those women who may be at high risk in developing the silicone implant problem, the Silicone Immune Dysfunction Syndrome. Knowing in advance that one is at high risk, it may be possible to stop the process before it causes significant organ system damage. Edelson suggests that since various studies of the immune system include natural killer cell activity, autoantibody profile, lymphocyte subsets, silicone antibodies and many other known factors, these factors, as well as organ systems, need to be looked at to evaluate the damage, and that knowledge, in turn, can lead to suggestions on how to go about repairing the body.
Unless the implants are removed from the body, such patients will have a continuing downhill course, and, often, simple removal may not be sufficient, as the healing process may require various treatment regimens including immune modulation, nutritional therapy for sensitivities, anti-oxidant therapy, environmental controls and psycho-neuroimmunological approaches.
How the Syndrome is Evaluated
Dr. Edelson2 and other physicians will evaluate Silicone immune Dysfunction Syndrome by the following steps:
- In depth history, including details of implant problems.
- Physical examination.
- Studies to rule out other conditions such as Lyme Disease, Multiple Sclerosis, etc.
- Various laboratory studies
- immune studies (complex)
- T-cell silicone immune study
- fungal or bacteriological studies (if indicated)
- biochemical profile
- skin testing (if needed)
- Address each organ system damage individually depending on patient's complaints.
- brain: PET, SPECT, BEAM. EMG, etc.
- pulmonary: PFT
- rheumatological: synovial fluid
- breast: MRI, Xeromammography
- GI: Digestive studies, pancreatic malfunction
- immunological: chemical antibodies, silicone antibodies, T-lymphocyte subpopulation, activated lymphocytes, lymphocyte immune function, natural killer cells and activity, immune complexes, complement levels, ANA, autoantibody analysis (myelin, striated muscle, thyroid, skin antibody, collagen antibody); skin testing (chemical, foods, etc.).
- Assessment of general damage to the immune system and direct damage from free radical oxidant molecules which has led to diffuse organ system damaging effects.
Therapy
During the course of therapy, many different, often unique, approaches are recommended, usually of one or another form of antioxidant therapy, coupled with proper diet, exercise, and other treatments.
Dr. Edelson2 has provided the following table to demonstrate various antioxidant factors or substances that protect against oxidant molecular species. Since, apparently, during silicone breast implant leakage, most, or all of the listed oxidants occur or add to the total body burden, treatment must be based upon relieving the load, and searchinb for ways of eliminating harsher effects.
| Free Radical/ Activated Species | Name | Antioxidants |
|---|---|---|
| O2 | Superoxide anion radical | Superoxide dismutases (SOD) |
| H2O2 | Hydrogen peroxide | Glutathione peroxidase Catalase |
| HO- | Hydroxyl radical |
|
| O= | Singlet Oxygen |
|
| PUFA | Polyunsaturated Fatty Acid Radical |
|
| ROOH | Organic/Fatty acid hydroperoxides |
|
| PR-S-SPR | Oxidized protein | GSH, sulfhydryl amino acids |
Dr. Stephen Edelson2 has also outlined the nature of the treatment protocol recommended for those with breast implant silicone leakage symptoms, as follows:
- Diet: organic, high alkaline, semi-vegetarian, moderately high protein
- Exercise: moderate low impact daily
- Intravenous nutritional therapy
- Detoxification
- Antioxidant
- Nutritional Supplementation -- Oral
- Immunotherapy
- Chemicals
- Food
- Silicon
- Inhalants
- Environmental and Chemical Controls
- Transfer Factor
- Dehyroepiandrosterone (DHEA)
- Immune Stimulants: thymus, herbals, etc.
- Intravenous gamma globulin
SBI Laboratories Antibody Tests
As reported in an abstract by Nir Kossovsky, M.D.,5 Consultant to SBI Laboratories, "Research has shown that silicone, an adjuvant, is a tacky substance that adsorbs the body's own molecules, a process that denatures the molecules6 and may cause them to look foreign to the body's own immune system.7 The adsorbed molecular layer then controls the biological response.8, 9, 10 The body may then engage in cytokine-mediated immune and autoimmune activity,12 which, in turn, may cause a wide range of symptoms.13, 14
"Determining whether any particular set of symptoms is the result of exposure to silicone requires, in part, a test that can detect antibodies that bind to the molecules denatured by silicone.15 Standard rheumatologic and immunologic tests for well established immune diseases may not detect the hypothesized nontraditional immune reactions caused by silicone exposure.12, 16, 17 SBI laboratories offers the DetecsilsTM Silicone Sensitivity Test as a research test to detect potential nontraditional antibodies....
"The body's biological responses to implanted medical devices such as silicone breast implants are directed principally by the concentrated and denatured proteins bound to the implant's surface. There are five potential clinical materials and their post-surgical surface film of adsorbed proteins,"5 as follows:
1. Clinically Imperceptible
"As implied, imperceptible symptoms are below the threshold of detection. However, while the inflammatory process may not be noticeable, the consequences of chronic inflmmation may produce secondary signs and symptoms. For Example, as a consequence of a local inflammatory process, the patient may perceive progressive hardening of an implant. Associated with the formation of an implant/ scar mass could be such symptoms as back pain, shoulder pain, and tightness of the chest."5
2. Local Inflammation
"Local symptoms would mimic those above, but would be more noticeable and may be associated with a greater degree of pain as well as tenderness, swelling, and heat. The skin may be slightly reddened secondary to the increased blood flow associated with inflammation."
3. Cytokine-Mediated Systemic Inflammation
"Cytokines liberated by the inflammatory cells in the tissues surrounding the implant are designed to act locally. However, when released in large quantities, cytokines may enter the circulation where they may have an effect on both the brain and the liver. In the brain, they can reset the body's thermostat, leading to fever and chills. In the liver, they can promote the release of acute-phase reactants that increase blood sugar and cause generalized muscle and joint aches and pains. These symptoms can be 'flu-like'."5
4. Systemic Immunological Activation
"With antibody production, symptoms may be identical to level 3, and although symptoms such as those in level 2 are also possible, our preliminary data suggest that local chest symptoms are less likely when there is antibody positivity shown by the Detecsil test. The biggest differences between level 3 cytokine activity and level 4 antibody activity are: first, permanent tissue damage induced when antibodies are being produced is more likely; and second, the symptoms are less likely to respond to medical therapy alone. Surgical intervention may be necessary, and even then, if silicone is still present in the body, chronic medical therapy may be required for clinical relief of the symptoms. Common symptoms associated with the anti-SSAA [anti-Silicone-Surface-Associated Antigens] study published in The Journal of Applied Biomaterial, December 1993, were analyzed recently. In that study, fever, sleep disturbances, and foot pain were found to be independently statistically significant symptoms (p<.05). Fever coupled with the absence of local chest pain was a statistically significant syndrome (p<.001)."5
5. Immunological Activation With an Autoimmune Component
"If the antibodies elicited have an autoimmune feature, the symptoms may be the same as in levels 3 and 4, but they may also include a wide range of neuromuscular and hematologic problems in organs that are distinctly separate from the breast or any region where gross amounts of silicone may have deposited. We do not have enough information to be more specific at this time."5
Future Research
Charles H. Farr, M.D., Ph.D.3 and other allied physicians have found that hydrogen peroxide infusions (IVs) will ease the pain, at least temporarily, of silicone symptoms.
Gus Prosch, Jr., M.D.,4 through use of ELF laboratory's Light Beam Generator,1 has discovered that swollen lymph glands will reduce. Whether or not a return of swollen lymph glands means that the body, under influence of the Light Beam Generator, simply recycles the tetramer and its products, or whether there is a net loss of silicone and its products has not yet been determined.
An overall body detoxification process developed by Lee Cowden, M.D. of Dallas, Texas, has brought about complete recovery from at least one lady who suffered from a combination of breast cancer and silicone tetramer leakage, as interviewed by this writer.
References
- Anthony di Fabio, Thomas Gervais, Courtland Reeves, Lymphatic Detoxification, 1994.
- Stephen B. Edelson, M.D., personal correspondence, lectures and publications, Environmental and Preventive Health Center of Atlanta, 3833 Roswell Road, Suite 110, Atlanta, GA 30342-4432, received 1994.
- Charles H. Farr, M.D., Ph.D., personal correspondence, 10101 S. Western Ave., Oklahoma City, OK 73139-2929, received 1994.
- Gus J. Prosch, Jr., M.D. personal communication, 1994.
- Nir Kossovsky, M.D., "A Capsule Summary of the Biological Response Triggered by Silicone Implants," SBI Laboratories, 1401 Forbes Avenue, Suite 237, Pittsburgh, PA 15219, received Janaury 1995.
The following references came from the abstract prepared by Nir Kossovsky, M.D., reference 5 above.
- Cheng SS et al. J Colloid Interfac Sci, 1994; 1662:135-143.
- Kossovsky N and Freiman CJ. Arch Path Lab Med, 1994; 118:686-693.
- Kossovsky N et al. J Biomed Mater Res, 1987; 21:1125-1133.
- Tang L and Eaton JW. J Exp Med, 1993: 178:2147-2156.
- Bonfield TL and Anderson JM. J Biomed Mater Res, 1993; 27:1195-1199.
- Naim JO and Lanzafame RJ. Invest Immunol, 1993; 22:151-161.
- Kossovsky N and Stassi J. Sem Arthritis Rheum, 1994; 24 (Suppl 1):18-21.
- Bridges AJ et al. Ann Intern Med, 1993; 118:929-936.
- Wilhelm K. Autoimmunity, 1993; 14:341-342.
- Bernstein RA. J Roy Coll Phys (London), 1990; 24:18-25.
- Kossovsky N and Papasian N. J Appl Biomat, 1992; 3:239-242.
- Kossovsky N et al. J Appl Biomat, 1993; 4:281-288.
Sources are given in references. Authors of contributions\quotations are alphabetically arranged; major author, if any, is in bold.
- Stephen B. Edelson, M.D.
- Charles H. Farr, M.D., Ph.D.
- Gus J. Prosch, Jr., M.D.
- Nir Kossovsky, M.D
- Anthony di Fabio, responsible editor/writer
Copyright 1994. All rights reserved by The Roger Wyburn-Mason and Jack M. Blount Foundation for the Eradication of Rheumatoid Disease AKA The Arthritis Trust of America.® The Rheumatoid Disease Foundation / The Arthritis Trust of America was dissolved in 2020 and all website content was transferred to the Foundation for Alternative and Integrative Medicine.