MycoplasmasFig1AFig1B.jpg
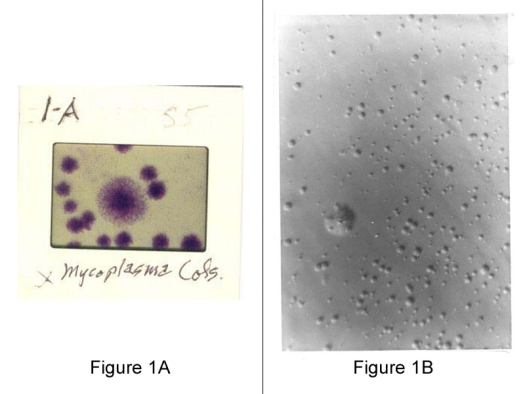
Image courtesy of The Arthritis Trust of America
Figure 1A (left): Slide of mycoplasma colonies as seen through a microscope.
Figure 1B (right): Agar plates is characteristic but not always typical and depends on both the culture and agar media as shown in this slide, from tissue cell culture.
Lecture I: Mycoplasmas Properties and their Role in Autoimmune Diseases
Thank you for inviting me to lecture on Mycoplasma Arthritis in humans.
Today I will review “Mycoplasmas Properties and their Role in Autoimmune Diseases,” by Harold W. Clark, Ph.D.
In 1952 when I joined Dr. Thomas McPherson Brown, chief of medicine at the George Washington University Medical School in Washington, D.C., as Research Biochemist, the chief of medicine at the University of Rochester in New York told me that Brown has a theory that some atypical microbe was the cause of Rheumatoid Arthritis that should be treated with antibiotics. Unknown to me, the year before, 1951, Brown and staff had just published and reported at the International Arthritis meeting their recent findings entitled “A Study of the Antigen-Antibody Mechanism in Rheumatoid Diseases.” This report emphasized the antibiotic treatment of rheumatoid diseases based on the suspected mycoplasma role in the inflammatory immune complex that should be blocked by immunosuppressing agents.
Because of its potential application the report was widely publicized in newspapers and magazines. For example the Associated Press reported “Hope Held out for Cure of Rheumatic Ills, Washington research team announces New Concept on Causes and Cures.” At that time except for the veterinarians, very few doctors knew what a PPLO or mycoplasma was, let alone its role in human arthritis.
Although Koch and Pasteur had brought bacteria to the forefront of medicine it was the microbiologists Nocard and Roux at the Pasteur Institute in 1898 that first isolated a filtrable bacteria from pneumonic and arthritic cattle. For the next 65 years new isolates were referred to as Pleuropneumonia-Like Organisms (PPLO), now known as Mycoplasmas in the broader Mollecutes family.
The terms PPLO and wall-less bacteria L-forms were used interchangeably until 1962 when a viral-like agent causing atypical pneumonia but sensitive to tetracycline and cultured in a cell-free media was found to be a new human mycoplasma strain (M. pneumoniae). Because of the increasing number of strains, PPLO were classified under bacterial Mollecutes with the mycoplasmatacae family and subspecies mycoplasma and ureaplasma. Our modern day culture techniques have improved over the early technique of implanting a cellophane bag with the specimen in a rabbit’s abdomen. The difficulties in isolating mycoplasmas from inflamed tissues still remains.
Since their first isolation, a major impediment to mycoplasma research and laboratory diagnosis of infection has been the difficulty of isolating mycoplasmas from the host tissues. To overcome this problem complex cell-free media are used for both isolation and cultivation. The media is usually based on beef heart infusion, peptone digest, yeast extract, serum and various supplements. The continued use of these complex undefined media components has limited the precise definition of mycoplasma’s metabolic pathways, genetic analysis, preparation of mycoplasma antigens free of media components and specific monoclonal antiserum free of endogenous antibodies. The ability to detect and identify mycoplasmas in tissues using the genetic Polymerase Chain Reaction (PCR) technique should be measured in relation to the host’s immunologic and other responses to an infection. In addition there is some concern about the adaptation of new mycoplasma isolates being changed when grown in artificial media and subjected to viruses.
The first question we should ask and define is “What are Mycoplasmas?” And what are their connections with human arthritis especially the chronic rheumatic diseases of unknown origin. If mycoplasmas are now known to cause arthritis in animals as well as acute and chronic disease in human lungs and the genitourinary tract their location in human joints could also be the irritant causing inflammation. Thus we have mixed tissue arthritis with a combination of symptoms. Mixed arthritis is further complicated by adding another vague term “Rheumatoid Arthritis” (RA). According to the U.S. Arthritis Foundation, the whole group of Rheumatic Diseases now constitutes 171 types of arthritis with associated diagnoses and mixtures of symptoms. These include mycoplasma arthritis, connective tissue diseases, and mixed connective tissue diseases. RA was initially called Chronic Infectious Arthritis, when it was first thought to be caused by some atypical viral-like agent and is now referred to as Autoimmune disease, Immune Complex disorders, and Collagen Vascular disorders. The diagnostic terminology is more confusing when based on the symptoms, the tissue targeted, the disease mechanisms, and the microbial causes. This terminology includes septic, reactive or infectious, bacterial, viral, Lyme, and mycoplasma arthritis in humans. The mixed symptoms caused by mycoplasmas are not the same as caused by bacteria and viruses. The different responses are attributed to mycoplasma’s different physical, chemical and host related properties.
What is Mycoplasma Arthritis?
The common symptom in most Rheumatic Diseases is inflammation as anatomically described by synovitis, bursitis, carditis, neuritis, or nephritis, etc. Many different causes of inflammation have been suggested and pursued. Upon further study and review, researchers may find that a common microbe, such as Mycoplasmas can cause various host responses or symptoms in similar tissues. Because of the different portals of entry differences between individuals may be observed. Because of the associative factors; age, gender, genetics, and environment a microorganism could cause arthritis in one patient, systemic lupus erythematosus (SLE) in another, or neither disorder. The same agent and mechanism that causes inflammation of the synovial tissues in a male patient may cause nephritis or both in a female. As in Juvenile Rheumatoid Arthritis, the juvenile host could respond differently to mycoplasmas than a mature host. Mycoplasmas could infect the fetus and lay dormant until challenged and later could cause different symptoms.
Of all the targeted organs and tissues, the blood supply is truly our lifeline and first line of defense that controls the severity and spread of viable agents. The blood supply may determine the primary site of disease activity, such as the kidney or liver, or synovium, and whether it is localized or widespread. Some targeted tissues such as the synovium, eyes, and kidneys are more vulnerable to the inflammatory disorders because they are more vascularized and present symmetrical symptoms. The composition and restriction of the blood supply by inflamed and swollen tissues becomes life-threatening if they control the blood pressure and volume. The potential role of mycoplasmas in the formation of arterial plaques and the subsequent host responses must also be considered. The availability of receptive tissues with attachment sites for a particular mycoplasma strain are essential for the host responses.
We should ask: How do Mycoplasmas cause Inflammation?
In 1983 the USA National Arthritis Advisory Board reported that “Because we know that mycoplasmas can cause arthritis in many animals and because we know that they do cause acute and chronic disease in humans (in lungs, and genitourinary tract) we must take seriously the possibility Mycoplasmas cause arthritis in humans.” As in arthritic animal models (pigs, rats, mice) the detection of viable mycoplasmas in arthritic patients “now you see them, now you don’t” has made “Hocus-Pocus” skeptics of many doctors when arthritis symptoms and mycoplasmas seem to hide and come and go with the weather. As in animal models mycoplasmas are difficult to isolate or recover even though their arthritis and serologic response continues.
MycoplasmasFig1AFig1B.jpg

Image courtesy of The Arthritis Trust of America
Figure 1A (left): Slide of mycoplasma colonies as seen through a microscope.
Figure 1B (right): Agar plates is characteristic but not always typical and depends on both the culture and agar media as shown in this slide, from tissue cell culture.
MycoplasmasFig2AFig2B.jpg
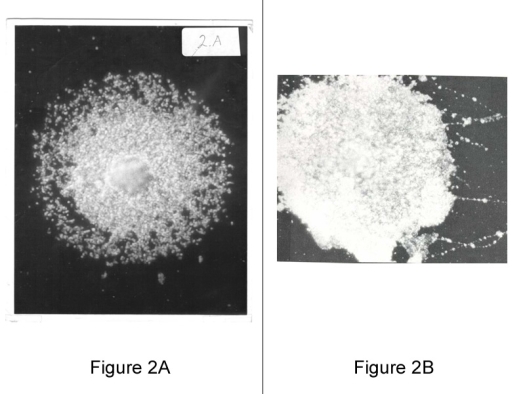
Image courtesy of The Arthritis Trust of America
Figure 2A (left): Mycoplasma Colony, Fixed on Glass Slide, 400X dark field. A dark-field photo of a mycoplasma colony showing the minimal reproductive particles.
Figure 2B (right): Dark-field photomicrographs of hot water-fixed Mycoplasma hominis, type 1, colonies, X 450. Variable large body and particulate chainlike filaments disrupted from colony. Shows the adhesiveness and pliability of mycoplasmas from a colony streaked on a slide producing filament like structures.
Most people know what arthritis is but only a few know about mycoplasmas, a unique microbial cause of arthritis in animals and in humans. Besides being the smallest free-living microorganisms, (between 50-100 nm), mycoplasmas without a cell wall are highly pleomorphic and likened to a viral size jelly-fish. Unlike the larger and more parasitic bacteria and smaller viruses, mycoplasmas are both saprophytic and parasitic opportunists living off the cellular debris as in tissue cell cultures or complex cell-free media.
MycoplasmasFig3AFig3B.jpg
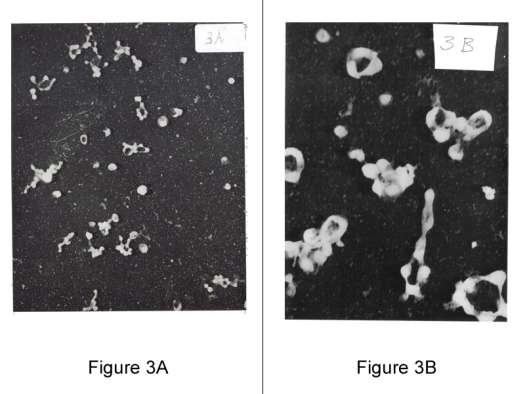
Image courtesy of The Arthritis Trust of America
Figure 3A: Electromicroscopic view of broth culture of Mycoplasma hominis, Type II, isolated from patient with Rheumatoid Arthritis X 20,000 magnification. Note bizarre, non-specific, poorly identified appearance of microbial elements.
Figure 3B: Published: Journal of Bacteriology 1965, 90, 1373-1386; Mycoplasmas – hexadic (6) budding.
Electron photomicrographs of shadowed mycoplasma specimens show donut (ring) shaped mycoplasmas at low and high magnification.
Rings or cherios, dented open from sedimentation, result in collapsed cell membrane. Mycoplasma cells appear flat when air dried. Culture of M.gallisepticum mid-stage growth, shows rings with 6 buds.
Similar morphology and division cycles were observed in 1935 by Dr. Nobel.
MycoplasmasFig4AFig4B.jpg
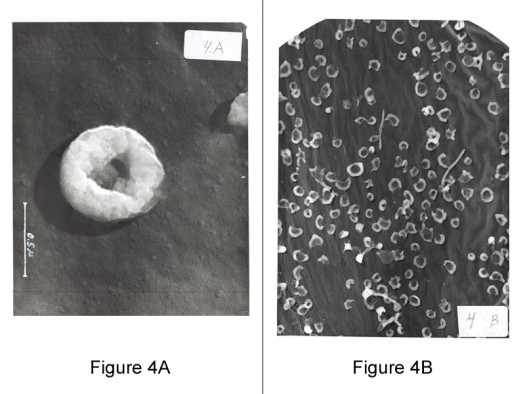
Image courtesy of The Arthritis Trust of America
Figure 4A (left): Mycoplasma: 100,000 magnification.
Figure 4B (right): Mycoplasma Culture: Electron Microscope
The three basic stages of growth with hexadic (6) budding of the minimum reproductive units, and with chains of budding rings.
The most significant observation of mycoplasma morphology and growth cycle is their hexadic budding into six viral size reproductive units that was reported in 1965, J. Bact. and again in 1985. This division may vary in other strains.
The basis for this hexadic budding was recently indicated by German investigators 1997, who were sequencing the genomes of two mycoplasma strains, pneumoniae & genitalium. They found that the genomes of both mycoplasmas could be subdivided into six equal segments. The other genes were well conserved within each of the individual segments but the arrangement of the six segments differed. This hexadic growth cycle now with a genomical basis should be pursued in the host’s cellular pathology and observed in the host’s tissues.
MycoplasmasFig5AFig5B.jpg
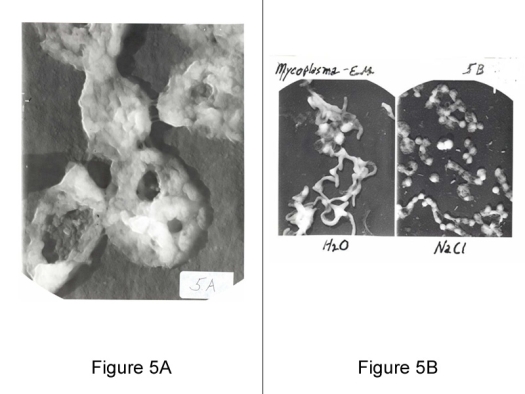
Image courtesy of The Arthritis Trust of America
Figure 5A (left): Five Mycoplasmas: 120,000 X magnification by electronmicroscope. High magnification showing membrane surface with adhesive attachment and infrastructure.
Figure 5B (right): Mycoplasma in water and salt. Mycoplasma’s sensitivity to low osmolarity are morphology and viability dependent, showing coccoidal in isotonic saline, and filamentous in water.
Mycoplasma’s minimal reproductive unit size as determined by ultrafiltration is less than 100 mu and dependent upon the strain and age of culture.
The filtration pressure could force larger pliable particles through the millipore membranes. With an estimated mass of 1.2 x ug. the smallest reproductive unit (bud) the genetic coding DNA would be approximately 4 x 106 molecular weight and suggests a limited synthetic capability (99% larger than 300).
Composition
In 1962 we were awarded a grant from the NIH to investigate mycoplasma’s chemical composition. This was the beginning of an endless problem. In addition to the wide ranging cholesterol composition we found the protein, nucleic acid, and lipid composition varied with changes in the tissue broth digest and serum enrichment. Little known and rarely reported, the basic peptides from tryptic tissue digests are essential for mycoplasma growth and apparently can reflect its tissue source. In addition the basic proteins such as IgG from serum enrichment was found to be incorporated by mycoplasma during In-Vitro growth. Thus it seems probable that mycoplasmas in the human host could incorporate and alter specific basic proteins in any number of tissues and form autoantigens but also act as their adjuvant carrier. A basic protein such as IgG becomes altered when attached to the mycoplasma carrier thus rendering it autoantigenic to the host. Native IgG or a basic protein is nonreactive to the human host unless attached to adjuvant. The autoimmune rheumatoid factor, anti-antibody, is only reactive to the IgG that is altered by attachment to a particle such as mycoplasmas, Rbc, or laytex. The specificity of basic peptides or proteins may depend on the mycoplasma strains with affinity to certain tissues indicated by their differences in other metabolic activity.
Digested broth extracts prepared from different tissues were found to equally support mycoplasma growth. The resulting mycoplasmas were found to have different composition and antigenic properties. Although the techniques available today could provide further details I believe the results would remain the same.
A marked difference was found in the phosphorus and nucleic acid composition of mycoplasmas cultured in a peptic digested beef broth (PD) and a tryptose digested casein-soy broth (TS).
A marked decrease in the DNA composition was found in mycoplasmas cultured in media containing penicillin. Non-inhibiting antibiotics penicillin and ampicillin appreciably decreased mycoplasma’s antigenicity when incorporated in their culture media. Other factors such as pH, and culture age could cause composition changes.
Mycoplasma’s antibiotic sensitivities are also altered. In another experiment using different media and strains the differences in media and mycoplasma composition were less marked.
Complement Fixation (MCF) titers in immunized guinea pigs showed mycoplasmas cultured in digested thymus broth more reactive and lung tissue specific.Variation in mycoplasma antigenicty was found in different culture media plus or minus antibiotics.
See Figure 6A (Table 6A): The mycoplasma complement fixation titers in sera from patients in a respiratory disease clinic was more reactive with mycoplasmas cultured in lung digest broth again indicating autoimmune reactivity and mixed infections. Low antigenicity in standard BH media. 117 positive for M. hominis.
| M. orale (Patt) | M. salivarium (PG-20) | M. pneumoniae | M. hominis | M. arthritidis | M. fermentons | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Media | B | P-lung | H-lung | B | P-lung | H-lung | B | B | B | B |
| CF positive | 2 | 6 | 13 | 11 | 10 | 0 | 2 | 17 | 6 | 0 |
|
Symbols:
* Harold W. Clark,Jack S. Bailey, and Thomas McPherson Brown, “Medium-dependent Properties of Mycoplasmas,” Diagn Microbiol Infect Dis, 3:283-294, 1985 |
||||||||||
The mycoplasma complement fixation titers in sera from patients in a respiratory disease clinic was more reactive with mycoplasmas cultured in lung digest broth again indicating autoimmune reactivity and mixed infections. Low antigenicity standard BH media. 117 positive for M. hominis.
MycoplasmasFig6B.jpg
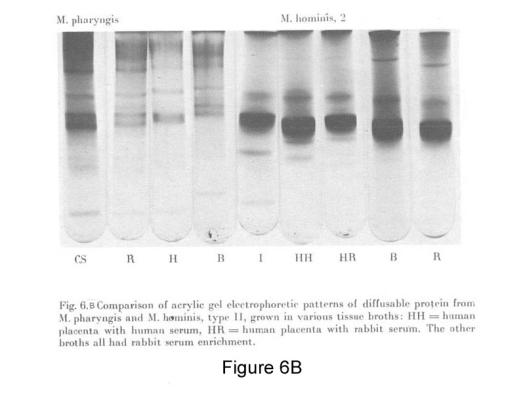
Image courtesy of The Arthritis Trust of America
Figure 6B: Disc-gel electrophoresis shows differences in protein composition of mycoplasmas cultured in different tissue digest broths from human sources.
Casein Soy and Intestine digest broths show different protein composition.
Each strain has its own antigenic pattern providing serologic specificity with polyvalent antiserum.
MycoplasmasFig7A.jpg
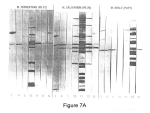
Image courtesy of The Arthritis Trust of America
Figure 7A: The antisera labeling of the SDS electrophoresis of mycoplasma antigens show M.salivarium antigen (11) preparation contaminated with common media antigens found in other strains. fermentans (10) & orale (12).
MycoplasmasFig7B.jpg
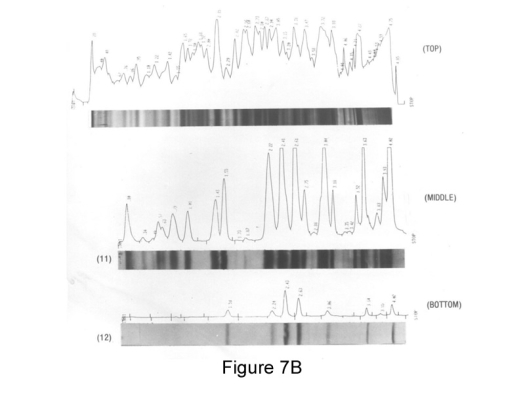
Image courtesy of The Arthritis Trust of America
Figure 7B: The laser scan of the SDS electrophoresis blot of M.salivarium, top: shows silver stained organic composition, middle: antigens reactive with antisera and bottom control showing non-specific media antigens observed in other strains.
Like their human and animal hosts mycoplasmas mimic their culture media, “they are what they eat.” For example mycoplasma’s cholesterol levels are elevated when grown in a high cholesterol media, that is essential for some strains as part of their lipoprotein membrane. Complicating research further, mycoplasmas mimic their media and change their properties depending on their localization in tissues: brain, lung, kidney, heart, synovium, In-Vitro. How much surplus food can and do mycoplasmas ingest? Mycoplasmas seem to grow equally in diluted (10x) and concentrated broth and more apt to colonize and infiltrate tissues with the highest degenerative rate. Unlike most inflammatory agents mycoplasma’s unique “Sleepy Virus” properties make them a prime candidate as persistent viable antigens and a causative role in different chronic autoimmune diseases; such as rheumatoid arthritis, lupus, diabetes, MS, etc.
What is your blood cholesterol level?
Mycoplasmas, like humans, not only require cholesterol for growth but can incorporate excessive concentration growing in a high cholesterol media -- such as a high blood level. What we don’t know but suspect is mycoplasma’s role in arterial plaque formation and the adverse effects of their elevated cholesterol levels.
Besides their specific requirement for basic peptides from tissue protein digests, mycoplasmas also require the nucleic acid precursors, purines and pyrimidines. These may be provided by the RNA and DNA nucleotides that have been degraded by the mycoplasma’s nucleases. Although mycoplasmas generally synthesize their own membrane phospholipids and lipoproteins from the exogenously provided fatty acids, some strains incorporate preformed host phospholipids into their membranes as another autoantigen. Being deficient in their ability to regulate the membrane fluidity by preferential fatty acid biosynthesis, the mycoplasmas overcome this difficulty by incorporating large quantities of exogenous cholesterol into their membrane.
Cell Membrane composition
Proteins constitute over two-thirds of the mycoplasma membrane with the rest being lipids. The membrane lipoproteins have attracted much attention since their abundance is remarkable in contrast to the limited number in eubacteria. Membrane lipoproteins are among the most dominant antigens in mollicutes and known to undergo antigenic variations.
Based on characteristic lipoprotein specific features, German investigators counted 46 lipoprotein genes in the M. Pneumoniae genome, while 21 putative lipoprotein genes were found in the M. genitalium genome. In this regard a lipoprotein extract from spinach has been used as the antigen to serologically test for M. pneumoniae antibodies indicating a broad range of lipoprotein antigens.
Virtually all mycoplasma lipids are located in the cell membrane and as in other biological membranes, consist of phospholipids, glycolipids, and neutral lipids that constitute a major portion of the hydrophobic core. Mycoplasmas are incapable of fatty acid synthesis and depend on the host or culture medium for their supply, including cholesterol. The dependence of Mycoplasmas on the exogenous supply of fattty acids and cholesterol has been one of their greatest advantages as models for membrane investigations. Thus the basis for the Osmium Tetroxide lipid fixation.
Due to the lack of a cell wall, the mycoplasma cell membrane is exposed to the external environment. This can facilitate direct contact of the mycoplasma membrane with its eukaryotic host, which could lead to fusion of the two membranes enabling the transfer and exchange of membrane components.
Thus the fusion or attachment of host membrane components is a potential source of antoantigens.
Energy Metabolism
Primarily because of their small size the mycoplasmas possess the minimum machinery for protein synthesis and growth. Consequently mycoplasma cell replication is much slower than in bacteria. Most striking is the lack of many energy-yielding systems. No tricarboxylic acid cycle, and no quinone and no cytochrome have been found in any of the mycoplasmas. The irony here is that after spending 4 years in graduate school investigating the respiratory cytochrome chain, I end-up spending 35 years investigating microbial pathogens that are cytochrome deficient. The electron transport system in mycoplasmas is flavin terminated. Thus high energy ATP is produced in mycoplasmas by substrate level phosphorylation, a less efficient mechanism than oxidative phosphorylation. Most strains are facultative anaerobes and antioxidants could impede their growth.
Some investigators have added high energy ATP to the culture media.
Summary of Mycoplasma’s Properties
The unique properties of mycoplasmas supports their role as etiologic agents in Rheumatoid and Autoimmune Diseases.
- Mycoplasmas are recognized as arthritogenic and systemic agents in animals and humans, with strain and species specificity.
- Colonization of both nasopharyngeal and genitourinary tracts by mycoplasmas is common, infecting four times as many women as men, a predilection in autoimmune diseases that predominantly affect females.
- Several strains isolated from the same and different animal species have marked physical, chemical and pathogenic variations.
- The pleomorphic characteristic of mycoplasmas, the result of the lack of a cell wall, their unusual growth cycles and growth requirements contribute to the problem of isolating and identifying mycoplasmas in host tissues.
- The low cytotoxicity of some strains of mycoplasma that have gone undetected in tissue cell cultures would support the finding of a low-titer antibody response and persistence of the mycoplasmas in symptom free hosts.
- Finding that the greatest incidence of mycoplasmal antibodies in humans associated with other infections would indicate that some mycoplasma strains are commensal or secondary invaders.
- Mycoplasmas are difficult to eliminate from their infected host with antibiotics and vaccines, a factor that indicates mycoplasmas persist fixed in or on tissues. Such persistence could account for the infrequency with which the organisms are isolated from host tissues.
- The rise in antibody titer to specific mycoplasmas and a delayed-type inflammatory response following trauma or therapy indicates the release of a persistent antigen, such as a Herxheimer reaction in a sensitized host.
- The characteristic of mycoplasmas, who’s composition mimics the culture media, could contribute to the production of autoimmune antigens, especially when the haptenic basic peptides required for growth induce tissue specific responses. Some strains of mycoplasmas are known to cross-react with different tissue substances as an autoimmune response.
- Localized and persisting mycoplasma antibodies are found in the synovial fluid of RA patients. The titers of circulating mycoplasma antibodies frequently are inversely correlated with the titer of rheumatoid factor (anti-antibody) .
- Mycoplasmas participate in several cell -mediated responses particularly the immunoinflammatory reactions of the delayed-type skin test.
- The immunosuppressive effect of mycoplasmas on the reactivity of peripheral T lymphocytes is comparable to the suppressor activity associated with autoimmune responses.
- Without a cell wall mycoplasmas are inhibited by a variety of substances used to treat RA, i.e. antimalarials, gold salts, bee venom, and tetracyclines.
Lecture II: Lecture on Recent Therapies of Human Mycoplasma Arthritis
Presented at the San Marcos University College of Veterinarian Medicine in Lima, Peru. (Summary)
What is mycoplasma arthritis and how do we effectively and safely treat the host?
Mycoplasma Arthritis – as defined by the Arthritis Foundation Primer on Rheumatic Disease, 10th Ed, 1993, “Mycoplasma pneumoniae infection is often associated with nonspecific arthralgias and myalgias. In some patients a migratory polyarthritis of medium size joints such as the shoulders, elbows, knees, and ankles may occur three to eight days after onset of initial illness and may last two months. In a few patients symptoms may last up to one year.” Mycoplasma arthritis is now listed as one of the 171 types of arthritis.
In the Beginning
Over 4 thousand years ago, in Egypt, the high priests treated Arthritic Consumption in the Pharoes with beer and bread made from moldy grain. Little did they know that the streptomyces mold on the grain and bread was a source of today’s most effective tetracycline antibiotics.
In today’s modern civilization of purified bottled water Egyptian women seem to have the highest incidence of Lupus, a severe autoimmune disorder and rheumatic disease also known as Immune Complex and Collagen Vascular disorders. Even though the causative agent may be the same, the symptoms of rheumatoid arthritis and other rheumatic diseases are not necessarily the same around the world. Most of our chronic diseases continue to persist while the causes remain unknown due to our genotypic differences. Having elevated medicine from willow bark to aspirins and from cinchona bark to quinine alkaloids, the failure of today’s health care, in desperation, seems to be returning back to the beginning with herbs and food supplements or back to nature. Many new symptomatic therapies have failed with time. Just as many of the infectious diseases, Tb, leprosy, and small pox were reduced in the world until something hidden like AIDS rises to the surface. We should also consider whether chlorinating and fluorinating our drinking water, while limiting some diseases may open the door for other diseases, like cancer, neurologic, and heart diseases. By eliminating the infectious cause of rheumatoid diseases we could also reduce the incidence of other diseases and premature deaths. Therapies that reduce or eliminate symptoms by inhibiting one oxidizing enzyme (Cox2) could also open the doors to the inhibition of other essential oxidases causing an adverse break in metabolic chains. Tissues smothered with excessive antioxidants may not survive and the over use of immunosuppressives can open doors for more severe infections like a double edge sword. Pulse therapy will allow the tissues to catch their breath, especially at higher elevations. Whereas the increased arthritis swelling and pain at that low pressure altitude could be attributed to diving down in the ocean where the increase pressure could reduce the volume or swelling and pain.
In view of the thousands of therapies proposed for arthritis the focus should be primarily on those aimed at eliminating and preventing an infectious mycoplasma cause and not just symptoms. Much like the Tb mycobacterium, mycoplasmas are difficult to isolate and eliminate even with multiple antibiotics.
A Mechanistic Treatment of Mycoplasma Arthritis
A mechanistic approach to the treatment of the infectious rheumatoid diseases can be more effective and less costly by controlling and preventing the disease and not just the symptoms. In addition to sustaining good health basically there are three targets:
- to SEARCH for and eliminate a persistent irritating microbial cause(s).
- to identify and BLOCK the antigen-antibody immune complex formation, and
- to control and ELIMINATE enzyme induced inflammation with tissue destruction.
The elimination of the microbial root cause should be the primary target. The less pathogenic or nonvirulent microbes should be less immunologically reactive and require less antibiotic treatment.
Three Prong Prescription >>> adjusted for each patient
- Antibiotics: such as minocyclines, in low pulsed doses should be directed at eliminating and inhibiting the atypical microbial cause. In addition the three-prong tetracyclines can also act as Antioxidants, Immunosuppressants, protein synthesis and metaloenzyme inhibitors.
- Immunosuppressants: such as prednisone, and many other chemicals that block the immune-complex formation that activates Complement proteolysis and further promotes tissue destructive inflammation.
- Antiinflammatory antioxidants: such as the non-steroidal anti-inflammatory drugs (NSAIDS and Cox-2) to eliminate and prevent the tissue destructive inflammation.
In addition the essential role of several major contributing factors affecting the occurrence and severity of the chronic arthritis and immunologic disorders must also be considered in selecting and benefiting from the most effective treatment. These include variable factors such as; physical and mental Health, Exercise, Nutrition, Allergies, Exposure, physical and mental Stress. The decisive fixed factors include: Age, Gender, and Genetic succeptability, all of which can help hinder or predispose the therapeutic success. These three therapeutic components are most effective when used collectively. The probes are only as effective as the recipient’s good health that can provide a unified basis for safe and effective therapy individually customized for each patient. Even the tetracycline’s lipophilic, nucleophilic and chelating properties that have positive multi-directional actions, must focus simultaneously on all and not just one action, as antibiotics can act differently in each patient.
Treatment: for Chronic Mycoplasma Arthritis
Intermittent dosing is important especially with drugs such as tetracylines that are multiple acting, like aspirin, and not just antimicrobial. With intermittent dosing there is less chance of causing resistant strains and toxicity. There is the precedent of other therapies, gold and methotrexate where weekly pulse doses were found to be less toxic and thus more tolerable and effective over time. Even the one day pause allows recovery of the host tissues from antioxidants, immunosuppressants, and protein synthesis inhibitor effects. Treatment is more effective chipping away over long periods with the eventual inhibition of the viable and less virulent mycoplasmas rather than overwhelm the tissues with drug toxicity. In most chronic diseases a drugs toxic actions on the host tissues is more critical than its effect on a benign causative microbe.
Chelated tetracyclines fixed in bone and other low metabolic tissues like cartilage provide a pool, replenished by intermittent dosage. Protein inhibitors’ excessive long term doses can damage organ functions and negate any benefits. Having to discontinue gold salts therapy after a few years is a good example. Antibiotic effectiveness is dependent on the tissue infected. For example mixed I.V. and oral dosages may be required to clear the fatty synovium tissue unlike the genital tract. In-Vitro studies found that mycoplasma’s antigenicity and antibiotisensitivity were variable when cultured in different tissue broths indicating the need for variable antibiotics and dosages. Bacteriostatic blood levels are not always critical when evaluating a multi action drug such as the tetracyclines.
Even with specific genetic coding the complex pathogenicity of mycoplasmas become a set of conditions that regulate the host-parasite or saprophytic interaction. A pathogenic substance such as mycoplasmas that cause a delayed or persistent abnormal host response in one set of conditions may be nonpathogenic in another set. Some strains of mycoplasmas and ureaplasmas are species specific for humans and animals and are specific pathogens for respiratory and genitourinary tract tissues. However in tissue cell cultures the mycoplasmas were found to coexist as cell bound noncytopathogenic saprophytes and remain untouched by antibiotic or antisera treatment. After 50 years of recognition mycoplasma’s continued persistence in tissue cell cultures indicate their resistance to therapeutic control in human tissues and the need for a special approach.
Another form of mycoplasma pathogenicity is their specific interaction with the host’s basic proteins such as IgG with their selective adhesion and conformation of specific cell membrane autoantigens. The cell bound immune complexes (IC) of antigen+antibody have been cited as pathogens for rheumatic diseases. An example is finding mycoplasma antigens in the IC extracted from rheumatoid synovial fluids.
The mycoplasma may have been transmitted in-utero and lay dormant until the host cells become receptive. The disease or the host response also depends on the pathogen’s portal of entry. The foreign antigens could elicit pathogenic host responses such as leukocyte activation resulting in antibody production and inflammation with tissue destruction. Also the attachment of mycoplasmas to blood cells, lymph vessels, and nerves could be responsible for plaque formation and impaired circulation as with the resulting agalactia restriction of milk in goats and the restriction of capillary fluids in plant and tree wilting diseases by the related citroplasma strains.
Mycoplasmas present a variable target as both extracellular and intracellular agents. With low cytopathogenicity, mycoplasma can remain attached to and lay dormant in tissue cells for months without killing the cells as long as the cells continue to shed nutrients. For years mycoplasmas (PPLO) were primarily considered nonpathogenic in humans even though veterinarians knew of their severe pathology in animals. Finding a closely related gorilla model with severe spontaneous rheumatoid arthritis and scheduled for euthanasia provided the ideal test subject. The model was an under weight five year male infected and serologically positive for a human Mycoplasma salivarium strain. He responded slowly as expected, initially with a Herxheimer reaction to the 1,000 mg IV doxycycline bi-monthly, resulting in less frequent and diminished flares. Over 4 years of treatment his weight tripled and he became a beautiful silver back gorilla specimen. Providing culture and serologic tests for mycoplasmas in captive gorillas in zoos around the world we learned of other arthritic gorillas who also responded to the I.V. tetracycline treatment.
Arthritogenic mycoplasma have been investigated and frequently reported in domestic animals such as; bovine, caprine, swine, rodents, avian, etc. Many others with species specific mycoplasma strains, including; canine, feline, equine, elephants, etc. have been isolated but seldom associated with their arthritis or antibiotic treatment. As in humans the experimental animal models show the difficulty in isolating or treating mycoplasma arthritis especially in the late stages.
Again I.V. antibiotic treatment has been found more effective than oral but more difficult and costly to administer.
Mycoplasmas, a.k.a. PPLO, were first isolated from a human ovarian abscess in 1932. Although strains are frequently isolated from the respiratory and urogenital tracts their isolation from synovial fluid and tissues has remained infrequent. Mycoplasmas and Ureaplasmas, a related species, have been isolated from and or detected in the broad spectrum of systemic tissues. Even though frequently associated with their host, identifying their role in arthritis or other rheumatic diseases still remains. Using today’s techniques the frequency of mycoplasma isolation and association has been greatly enhanced..
Ureaplasmas have been found associated with natural and experimental inflammatory disease in both animals and humans. In humans NG urethritis and arthritis are the primary symptoms associated with some strains of ureaplasmas. Because of their similarities mycoplasmas could be considered a mixed infection identified by a mixed culture with tiny ureaplasma colonies. In addition to arthritis, respiratory, neurologic, and urogenital diseases, ureaplasmas and mycoplasmas have also been found associated with spontaneous abortion, infertility and low birthweight in both animals and humans that have frequently been controlled with tetracycline therapy. Ureaplasmas have also been found in the fetal brain and spinal fluid, and from children of all ages as well as adults, especially those sexually active and child bearing age. The appearance of rheumatoid arthritis following the delivery of the second child could indicate a delayed hypersensitivity response to previous contact and following an immunosuppressive period. Thus in some cases mycoplasma could persist as a slowly developing irritant finally surfacing years later in chronic inflammation symptoms of arthritis and other autoimmune diseases. Unless the host’s immune defense system is mature and healthy and brings the mycoplasma infection under control, additional help with antimycoplasma therapy should be prescribed for both their control and prevention at the earliest possible time.
The clinical spectrum of M. pneumoniae diseases is far from complete. Originally thought to be a unique respiratory virus specific for humans and hamsters it has been associated with musculo skeletal arthritis as well as neurologic G-B syndrome, hematologic and respiratory diseases. To be most effective antibiotic treatment is given at the early onset of pneumonia as in rheumatoid arthritis. Even after treatment M. pneumoniae is shed indicating a hidden source of infection. Being hemolytic and reactive with specific red blood cell membrane antigens M. pneumoniae elicits cold agglutinin antisera and autoimmune reactions.
Based on positive serological blood tests females were found to be infected with mycoplasmas 4 times more frequently than males, a prerequisite to mycoplasma’s role in the female dominant autoimmune rheumatoid disorders. Persons with greater exposure especially the females were found infected more. Using several mycoplasma strains our lab found clinic patients up to 80% positive.
Even though the viable mycoplasmas are frequently transmitted among families, arthritis in humans usually is not contagious. However, because of possible family contact and mycoplasma transmission, antibiotic treatment of the family should be considered especially if they are both infected. As in other chronic diseases the genetic or family susceptibility as well as poor health and stress all contribute to the host’s symptoms and response to treatment. The apparent increase in female susceptibility to mycoplasma infection may also indicate a need for higher antibiotic dosage in treating mycoplasma arthritis in females. Unfortunately when the mycoplasmas acquire components and properties from the host’s tissues they become less of an irritant and as an autoantigen result in abnormally weak immune responses that are more difficult to measure.
Four years ago several clinical trials confirmed that the antibiotic minocycline was a safe and effective treatment of rheumatoid arthritis. These results supported the suggestion the late Thomas McPherson Brown, M.D. made in 1949 that antibiotic therapy may be beneficial for some people with RA that was caused by an infection with a microorganism called a mycoplasma in the joints, and that oral and/or intravenous antibiotics should be used in the disease’s treatment.
The book “Why Arthritis?” by Harold W. Clark answers the questions, “Why has it taken over 40 years to test and demonstrate antibiotic’s effectiveness?” and “Why withhold a safe and effective treatment when nothing safer and more effective is available?” Although the 48 week clinical trial of oral minicycline on two hundred rheumatoid arthritis patients was found to be safe and effective there apparently was no attempt to measure the occurrence of any infectious agents before or after treatment. Several clinics world wide have shown minocycline treatment both safe and effective in the treatment of rheumatoid arthritis. In view of this it is discouraging to see the Arthritis Foundation list antibacterial treatment under “Questionable Therapies” together with many unproven remedies in their current 1999 Primer on Rheumatic Diseases. Minocycline is listed as a disease modifying antirheumatic drug (DMARD) but not recomended for treatment. A five year comparison of the effectiveness of the approved gold salts therapy with the much less toxic antibiotic therapy is illustrated in Figures 8 & 9.
When Mycoplasma Arthritis is listed as one of the 171 types of arthritis, antibiotic treatment should be essential and not questionable. Obviously any infection associated with arthritis, in view of its unknown cause, should be treated with antibiotics especially if it has been proven safe and effective. Failure to test for and identify Mycoplasmas or other infectious agents does not exclude their causal role in rheumatoid or autoimmune diseases. Also the safe and effective response to antibiotic therapy does not prove an infectious cause. Unlike mycoplasma arthritis, syphilitic, tuberculosis, or gonococcal arthritis are highly contagious systemic diseases where arthritis is only one of the many symptoms that require special therapeutic control.
Why test for Mycoplasmas?
Some atypical viral-like infectious agent has long been suspected of causing chronic rheumatic diseases but rarely tested with antimycoplasma treatment. Testing and finding mycoplasma, although important, is not easy and requires a research lab with special skills to do acceptable tests. Testing for mycoplasma antibodies and antigens can be done three ways: by culture, Polymerase Chain Reaction, or serologic. Patients can have multiple infections, many of which may be involved in the rheumatic disease process. The multiple strains of mycoplasmas including the ureaplasmas can respond to different antibiotics. If both strains are present 2 antibiotics should be given to eliminate both strains. As mentioned tetracycline and other antibiotics have multiple actions that can give relief of symptoms independent of their biostatic sensitivity levels. A positive test for mycoplasmas or other microbial infection requires the appropriate antibiotic treatment for the primary diagnosis and arthritis as the secondary diagnosis.
Many of the rheumatoid diseases are now recognized as immune complex (IC) disorders, the result of binding microbial antigens with their antibodies.
The IC formation, fixed in tissues, activates the complement cascade of proteolytic enzymes causing synovitis, vasculitis, dermatitis and glomerulonephritis. What is still not recognized and accepted is the multi action of tetracycline antibiotics as immunosuppressants in blocking the IC formation. Except for their toxicity other beneficial medications, (NSAIDS,cortisone) could have a similar but limited effects.
Non-Responders
Although many patients with various rheumatoid diseases have responded well to long-term antibiotic therapy, there are some who do not respond or stop. There can be several reasons for a patient’s failure to fully respond. Some of these include: Some generic brands do not work at the same dose and frequency as the drug tested in original trials. Other pre-existing conditions and medications may hinder the response to antibiotic treatment. The patient’s immune system may be deficient or stressed caused by a concurrent infection or condition limiting the attack on mycoplasmas. The patient is self prescribing over-the-counter medications that are contra indicated for use with tetracyclines, such as vitamins, minerals and antacids. Also hormonal or metabolic imbalance could interfere with antibiotic action. Some strains of mycoplasmas may be found to be resistant to some antibiotics requiring a change to one more sensitive. Poor gut absorption may limit medications effectiveness. The inflammation may cause a barrier to drugs effectiveness requiring a constant supply of anti-inflammatory (NSAIDs) medication. After years of long term antibiotic therapy a patient could become tolerant of the antibiotic requiring a change. The possibility of a recurrent exposure to mycoplasmas must be eliminated. The dosage and antibiotic must be adjusted, higher or lower for each patient over time to achieve maximum benefits. Intravenous and oral treatment may be required. Long term therapy response is usually slow and gradual with rate and extent of improvement depending on the duration and severity of disease. The patient’s response to medication is very individualized, male and female, young and old, with treatment adjusted frequently to accommodate their needs.
In Conclusion
I will leave it up to the patients and their doctors to decide whether treatment should include searching for and treating an infectious immunological cause and not just the continued palliation of symptoms.
From the book: “Why Arthritis? Searching for the Cause and the Cure of Rheumatoid Disease” by Harold W. Clark, Ph.D.
San Marcos University College of Veterinarian Medicine Lima, Peru Lectures, August 1999 by Harold W. Clark, Ph.D. Mycoplasma Research Institute.
All rights reserved by the The Roger Wyburn-Mason and Jack M.Blount Foundation for Eradication of Rheumatoid Disease AKA The Arthritis Trust of America®
The Rheumatoid Disease Foundation / The Arthritis Trust of America was dissolved in 2020 and all website content was transferred to the Foundation for Alternative and Integrative Medicine.


