DeuteriumDepletedWaterMolecules.jpg
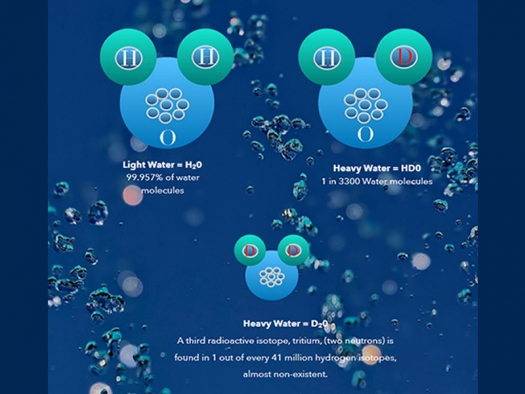
Image courtesy of Litewater Scientific
It was 13.8 billion years ago... In the first sixty seconds following the Big Bang, in the primordial plasma soup that was to become our Universe, the first element was created. That element was hydrogen, made simply of one proton and one electron.
The temperature was 1 billion degrees. Electrons and positrons annihilated to make photons, while protons and neutrons combined to make deuterons, a proton and neutron pair. Almost all of the deuterons then combined to make helium. Primordial matter was thus separated into two unequal parts: one quarter helium, three-quarters hydrogen. The process, however, was not completely thorough; as the universe cooled a small percentage of deuterium nuclei remained as they were, unpaired and isolated, trapped between hydrogen and helium. Most became the source of star energy along with hydrogen. The deuterons that survived the stellar furnaces eventually combined two-to-one with oxygen atoms to create water, now found in seawater and fresh water on Earth. There are about 6 drops (300 milligrams) of deuterium in each liter of water.
Fast forward 13.79999 billion years to 1932 when Harold C. Urey and his colleagues Ferdinand G. Brickwedde and George R. Murphy at Columbia University proved the existence of deuterium. Before their discovery it was believed hydrogen contained one proton and one electron. This rare heavier isotope of hydrogen with an added neutron doubles the weight and has a mass of 2. Deuterium had gone undetected by physicists perhaps because it only made up 0.0149% of all hydrogen in the Universe, or 1 heavy water molecule for every 3300 of regular water molecules in seawater. Some physicists suspected the existence of a second isotope of hydrogen as early as 1913, and Urey was keen prove it true once and for all. With certainty, he knew it must exist because something between the atomic weight and the mass spectrographic value of hydrogen simply did not add up. And with Brickwedde’s help at the Cryogenic Laboratory at the National Bureau of Standards they unlocked the mystery and proved its existence. The excitement of what they had discovered created a brisk back and forth on what to name it: barogen, haplogen, diplogen, deutium and dygen were suggested. Eventually they settled on deuterium (from the ancient Greek deúteros meaning second). To single proton hydrogen, that makes up 99.98% of all hydrogen they gave the name protium. In 1934 Dr. Urey won the Nobel Prize in Chemistry for this monumental discovery that would usher in the atomic age. Concentrated deuterium, i.e., Heavy water was the missing piece needed for nuclear reactors and the making of atomic bombs. It can be stated that because of Urey, Brickwedde, and Murphy, the world would never be the same.
While the 30’s brought a paradigm shift in physics, much was the same with biology. In 1929 ATP, the fuel of all life was discovered. This came 39 years after the discovery of mitochondria, where ATP is created. In 1937 the Krebs, (or Citric Acid Cycle), was discovered, describing the mechanism by which ATP is produced. But it would have to wait another 60 years until deuterium’s effect on ATP production would be understood.
Urey’s mentor Gilbert N. Lewis, professor of chemistry at Berkeley, was the first to create pure heavy water via electrolysis in 1933 shortly after Urey proved its existence. And subsequently he was the first to observe that this heavy water when frozen sank instead of floated like normal water. He also observed it strongly delayed the reproduction of microbes and retarded the growth of seeds.
A new era of research, focusing on this newly discovered hydrogen isotope, deuterium, was ushered in. Soon after professor Lewis' heavy water observations, Oscar W. Richards, a researcher at the Osborn Zoological Lab at Yale University showed that the splitting process of sugar with yeast is nine times slower in heavy water.
From 1934 to 1939 H.G. Barbour and his colleagues from the Pharmacology Department at Yale initiated the first systematic study on the effects of heavy water on mice. Between 1933 and 1939 there were 216 published English language studies on the biological effects of deuterium, all arriving at the same observation: heavy water was an impairment on life. The experiments replacing normal water with just 30% heavy water caused bacteria, plants, and animals to die in a matter of days. As World War II approached, heavy water became increasingly difficult to obtain for further because of the great demand in the military sector. Biological research on deuterium was stifled and gradually faded away until the 1950’s.
It was about the same time Francis H.C. Crick and James D. Watson announced the double-helix structure of DNA in 1953, when a gerontology and genetics graduate student named Gennady D. Berdyshev at the University of Tomsk in Siberia (Soviet Union) was urged on by a colleague, Boris N. Rodimov, a biophysicist, to investigate a very peculiar anomaly concerning lifespans of the Soviet population. While the average percentage of centenarians in all of the Soviet Union was less than 50 per one million, in certain areas of Siberia there was a striking number of centenarians – 324 per one million people, and furthermore, most of the population of Altai and Yakutia enjoyed great health and vitality well into their old age. Knowing that these regions were uniquely provided with pristine glacial-melt water from high altitudes, he was motivated to investigate this factor as a possible common denominator of the longevity of their inhabitants. Scientists focused on the possibility that some unique and unrecognized water characteristic might be involved - a mystery hidden perhaps in ancient glacial ice. The first experiments consisted of mining permafrost at a depth of 20 meters and melting water that had lain as ice for 300 million years. In the lab, it was observed that water stimulated cell division and slowed down aging. When the institute could no longer pay for the extraction of ancient ice they evaluated Siberian snow from their own vicinity, and to their surprise it had a similar effect. The theory of deuterium depleted water was beginning to take shape.
The experiments done by V.M. Muhachev at the Tomsk University in 1959 to 1960 convinced his colleagues that even a small dose of deuterium distorted the chemistry of hydrogen bonding and inhibited sub-molecular processes. By 1960 Berdyshev had enough information to conclusively link the longevity of the Yakuts and the Altaians with the consumption of glacial melt water. The researchers from Tomsk discovered that ancient ice, high latitude mountain snow, and glacial runoff were 15-20% depleted in deuterium compared to what became known as the Vienna Standard Mean Ocean Water (VSMOW), which is 155.76 ppm at the equator. No sooner had Berdyshev, Rodimov, Muhachev, et., al discovered this rejuvenating water, that a Level 6 nuclear disaster occurred at the Kyshtym nuclear power plant in the southern Ural Mountains, the third largest nuclear disaster in history! Berdyshev and his colleagues provided their newly discovered “miracle melt water” to a number of the victims and they were saved. It wasn’t until after the Soviet Union fell that the Russians declassified the disaster as well as how deuterium depleted water was used in medical treatment.
In 1966 Rodimov and his Biophysics Department chair I.V. Toroptsev were allowed to publish their work in English for the benefit of researchers and scientists everywhere. With their groundbreaking findings in “Biological Role of Heavy Water in Living Organisms” they put Tomsk on the map. They became the very first scientists to show how water depleted in deuterium had a positive biological effect. In the mouse experiments, they observed the increase of heavy water to 3% caused offspring to have a 20% lower birth weight, 3X smaller adult size than the control group, and the inability to reproduce a third generation. In another experiment, mice consuming glacial melt water had greater sexual activity and grew faster and bigger than the control group. These experiments were repeated in many Soviet institutions with different animals and plants. Considering that deuterium had only been discovered 30 years before, this was a monumental breakthrough. A secret of longevity had just been revealed!
Coincidentally, around the same time, one of the greatest revelations in biology was taking shape by Paul D. Boyer, a molecular biologist with UCLA. He discovered that tiny protein nano-motors within the mitochondria, sitting at the end of the Electron Transport Chain (ETC) bore the final burden for creating ATP. This protein assembly, spinning at 9000 RPM, has the structure and function of a mechanical motor, complete with rotor, stator and magnetic field. Boyer christened it “ATP Synthase”. It would be another 40 years, and the turn of the millennium, before deuterium’s effect on ATP Synthase would be uncovered.
By the early 1960’s it was clear that deuterium, although a hydrogen isotope, was something altogether “different” both biochemically and biophysically, being twice the mass of protium, and adding a neutron to the mix. No other element has such an extreme difference in mass among its isotopes. The understanding of how deuterium functions at the cellular level was yet to be discovered.
While the Russians were doing their research and making quiet breakthroughs, Americans were also hot to blaze a deuterium trail. It was 1963 when John F. Thomson of the Medical Research division of Argonne National Laboratory in Illinois wrote the definitive 152-page treatise entitled “Biological Effects of Deuterium.” The work of his colleagues Joseph J. Katz and Henry L. Crespi reinforced the biological implications of deuterium, noting early on in “Deuterated Organisms Cultivation and Uses,” published in 1966 that deuterium affects the shape of proteins and the replication of DNA. Laboratory mice experiments were conducted in which their normal body water was altered in the percentage (%) of heavy water, yielded the following results:
- Experiment #1: Laboratory mice body water was increased in concentration of heavy water to 30%. It proved to be fatal to the mice in a matter of days.
- Experiment #2: Laboratory mice body water was depleted in deuterium by 30% (105 ppm), resulting in significantly increased lifespan.
Ten years later in 1974, again at Argonne National Labs, British scientist T.R. Griffiths, at the 2nd International Conference on Stable Isotopes, proposed the theory that deuterium might be the primary cause of aging. In “Possible Roles of Deuterium in the Initiation and Propagation of Aging and Other Biochemical Mechanisms and Processes” he states, “Deuterium adversely affects the shape of enzyme molecules which are involved in DNA replication.” He observed that deuterium being more electronegative than hydrogen, twice as heavy, and having different atomic binding properties than normal hydrogen (protium), interfered with DNA replication. Because DNA repair enzymes contain deuterium in a position reserved for protium, they have a potential for participating in an error reaction, thereby compromising DNA replication and repair. The following year, in 1975, J.D. Gleason and I. Friedman, replicating the Russian findings on plant growth, published the first American study on using deuterium depleted water (DDW) to increase the growth of grains. This small but significant publication in NATURE magazine paved the way for a new generation of scientists to try and understand deeper the function of deuterium in the biology of living things.
When the Hunza people of northern Pakistan were investigated for their increased longevity and lack of illness it was determined that the deuterium content of their water, from the glaciers of Mt. Ultar, was about 133 ppm, a deviation of 16% from the 155 ppm global standard. A 16% reduction may not seem significant, however, Griffiths’ theory further predicted that the adverse biological effect of deuterium is proportional to the square of the concentration. And that is the reason we now know that even a slight depletion of deuterium has a great biological benefit.
By the 1990’s pivotal research was being furthered in Romania and Hungary. W. Bild and colleagues at the Romanian University of Medicine and Pharmacology showed that mice exposed to a sub-lethal dose of 8.5 grays of radiation had a greater survival rate on deuterium depleted water. Mice consuming water that was reduced to 30 ppm of deuterium had a 61% survival rate whereas the control group consuming plain tap water (150 ppm) had a survival rate of only 25%. The test group also maintained normal white blood cell and red blood cell platelet counts as compared to the control group which did not. The same two groups of unfortunate rodents were also infected with pneumonia and the test group showed an intensification of immune defenses not seen in the control group. The scientists concluded that mice with lower levels of deuterium in their systems would benefit from less error prone cell division and more effective repair of radiation damaged DNA. It was proof yet again that deuterium depleted water had some unknown and seemingly miraculous biological effect. These animal tests were carried out for the sole purpose of evaluating the effects of deuterium depletion for patients undergoing chemotherapy.
This, along with the work of Hungarian Nobel-prize winner Albert Szent-Gyrgyi, inspired the work of Gabor Somylai, a doctor and molecular biologist who in 1991 undertook the most extensive clinical trials of deuterium depletion yet completed, his data published in 1998, in the paper “The Biological Effects of Deuterium Depletion” and his 2001 book “Defeating Cancer.” Somylai’s double-blind clinical trials showed first that deuterium depleted water was free of any side effects and second, the survivability of his test group was significantly better than those cancer patients in the control group. He showed that consuming deuterium depleted water was an excellent complementary adjuvant to conventional radiation and chemotherapy. Between October 1992 and the spring of 1999, Dr. Somylai and his team administered some 350 tons of deuterium depleted water to approximately 1,200 patents generating over 12,000 pages of documented records. His groundbreaking work put Hungary on the map as an important center for research on deuterium depletion.
After the fall of the Soviet Union, Berdyshev, by now a famous gerontologist and chair of the genetics department of Taras Shevchenko National University in Kiev, started the new department of Juventology. His work no longer a sensitive national security matter, he and his colleagues built three industrial machines to recreate glacial melt water able to reduce deuterium levels by 30-40% (90-105 ppm). These engineering masterpieces separated deuterium out of water using freeze and thaw cycles, taking advantage of the fact that heavy water freezes at a slightly higher temperature. It was also publicized that people without access to deuterium depleted water could do something similar at home calling it the “Melt Water Cure”. The instructions called for performing repeated freeze and thaw cycles using a household freezer. By removing the water that freezes first repeatedly, a 5% reduction was claimed to be achieved. This protocol became very popular in Russia and many other countries from the 1990’s until today. By the beginning of the 21st century it was well understood among researchers that consumption of deuterium depleted water protected DNA from damage. But it was not yet known how. Thanks to man’s insatiable desire to uncover the mysteries of life it was only a matter of time until this puzzle would be solved.
In 2006, Russian chemist Igor A. Pomytkin and his colleague O.E. Kolesova published the study “Relationship between Natural Concentration of Heavy Water Isotopologues and Rate of H2O2 Generation by Mitochondria.” They were able to show that whatever mechanism allowed heavy water to damage the cell and vice-versa allow light water to keep it healthy was happening somewhere within the mitochondria. The study showed that heavy water inhibited mitochondria’s ability to produce hydrogen peroxide (H2O2) which acts as messenger molecules sending signals to regulate oxidative stress. Pomytkin’s work would lead him to the same conclusion about deuterium’s effect on ATP as was published the following year.
2007 marks the occasion of the most monumental discovery in the short history of deuterium science. Abdullah Algun, a medical doctor, biochemist, and pharmacologist from the Department of Biochemistry and Clinical Biochemistry at Gülhane School of Medicine in Ankara, Turkey published the paper “Biological Effects of Deuteronation: ATP Synthase as an Example.” It was shown for the first time how deuterium caused its damage in the last step of the Electron Transport Chain, within the ATP Synthase nano-motor. Algun determined that roughly every 15 seconds a bare deuteron (a proton – neutron pair), for which there is no open receptor, impinges on the fast spinning nano-motor, causing it to jam, stutter and ultimately break down. Algun further explains in the paper “Deuteronation and Aging,” published the same year in the Annals of the New York Academy of Science that this is the primary cause of aging! The mystery of how deuterium damaged life was finally revealed! The Nobel Prize worthy significance of Algun’s findings may be hailed as one of the greatest discoveries of the 21st century and one of the greatest oversights in biology.
At the time, only a handful were keen to take notice and register the gravity of this breakthrough. One person that got the picture right was Anton Chernopiatko, a Russian businessman, scientist and deuterium depletion enthusiast who co-authored with Pomytkin and Oxford scientists the 2015 study, “Deuterium Content of Water Increases Depression Susceptibility: The Potential Role of a Serotonin-Related Mechanism.” Having embraced the importance of deuterium depletion from an early age, a lifelong quest was about to manifest. Chernopiatko, now having definitive proof of deuterium’s role as a biological Trojan horse, took over the task to advance light water production beyond laboratory and research purposes and construct a factory to produce light water on a commercial scale.
Whereas Berdyshev in the 90’s had created an industrial process for reducing deuterium by 30-40% using a refrigerated process, it was 2003 that the first vacuum rectification column exclusively for the production of light water was developed at the Institute of Fine Chemical Technologies in Moscow. This was a much more efficient process that could remove 97% or better of heavy water. In 2012, Chernopiatko began construction of a plant in the Russian countryside commercializing the technology developed at the Institute in Moscow. A decade of prototyping, industrial engineering, hard work and luck yielded the first dedicated facility in the world to produce 90%+ DDW continuously.
It is 2019; the mechanisms of deuterium and how to remove it are well understood, yet there is still so much more to be learned. And the awareness of this colossal discovery is still in its infancy. Three international conferences on deuterium depletion in Budapest have provided a place for scientists to present their work. Laszlo Boros, MD and professor at the University of California, is an American scientist advancing the work in this field. He is the co-founder of the Center for Deuterium Depletion in Los Angeles, where deuterium testing and treatment protocols are offered to the public.
DeuteriumDepletedWaterGlassIceCubesMountains.jpg

Image courtesy of Litewater Scientific
The author and other scientists share the view that the ideal deuterium level for the body is below 120 ppm. Achieving this requires drinking one and a half liters of 100 ppm DDW daily for 2 months, minimizing the intake of all other beverages and maintaining a modified ketogenic diet with intermittent fasting and targeted photonic stimulation.
At the moment, there are three primary producers of deuterium depleted water, Preventa in Hungary, Qlarivia in Romania, and Vividi in Russia, whose production is offered in the United States as Litewater, the exclusive and only deuterium depleted water available in the world where 94-97% of the deuterium is removed.
As one would expect when something is too good to be true there is a catch and that is the shortage of supply. The extraction of these six drops of heavy water per liter is a complex multi-level technical process requiring special large scale industrial installations of specialized equipment. Unfortunately, it is not a machine you can have at home. In the future, as awareness and demand increases, new production plants will come online, and ever more efficient DDW technologies will be developed. China, Japan, Korea and other countries are actively pursuing their own research for heavy water separation, In the ninety years from the time hydrogen’s second isotope was uncovered until now, the world has changed unlike at any other time in human history. We stand at the dawn of an age of technology and the gateway to a quantum leap in biology. Thanks to the pioneers herein, radical life extension has a powerful new ally.
For more information visit the Litewater Scientific website.
A Brief History of Deuterium Depleted Water was originally published July 31, 2019 by Litewater Scientific; used with permission.

