MesenchymalStemCells.jpg
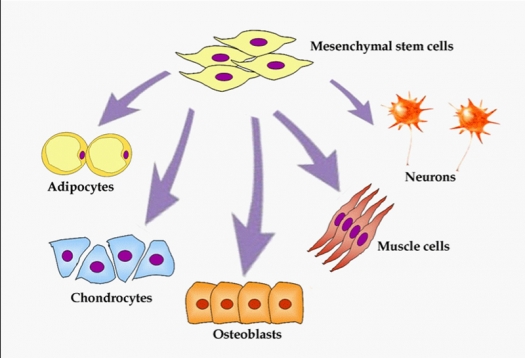
Image from Meregalli M, Farini A, Torrente Y. Mesenchymal Stem Cells as Muscle Reservoir. ©2011 / CC BY 2.0
Mesenchymal stem cells can differentiate in a large variety of human tissues including osteogenic, chondrogenic, adipogenic and neuronal lineages.
There are four different types of lupus erythematosus, a group of diseases commonly referred to simply as "lupus." Systemic lupus erythematosus(SLE) is the most common and serious form.
Professor Lingyun Sun and his colleagues have written five key articles about the treatment of Systemic Lupus Erythematosus (Lupus) with mesenchymal stem cells. I had the pleasure of meeting Professor Sun on a trip to China in 2010. Dr. Sun is at Nanjing University Medical School, in Nanjing, China.
The first of the series1, published in 2009, firstly described benefits of mesenchymal stem cells (MSCs) in a mouse Lupus model. From the positive outcomes they saw in that model, they then went to humans and treated a series of 4 people who were not responding to treatment with cyclophosphamide and steroids for more than six months. Both the mice and humans were treated with donor bone marrow mesenchymal cells that were grown in the laboratory. The lupus patients received a single intravenous (IV) infusion of 1 million cells per kilogram of body weight. For an average sized person the dose would have been 70 million cells.
The patients were tapered off of their cyclophosphamide during the six months following the treatment and remained on a relatively low dose of prednisilone (10 mg per day) for six more months. The net result was that the patients' kidney function, survival, and disease remission improved in the MSC treated patients.
Also of note from this study was that the Lupus patients' own bone marrow MSCs were cultured and they were found to be impaired compared to matched controls. The particular impairment was in the cells' capacity to form bone. They describe this impairment as "an osteoblastic niche deficiency." This deficiency comes along with impaired ability to produce T-regulatory cells – key cells for keeping the immune system in check – cells that are often decreased in number in patients with autoimmune diseases.
The second article2 in 2010 followed 15 Lupus patients who were treated with again bone marrow MSCs that were cultured in the laboratory. To be included in the study patients had to have severe SLE. To qualify as severe they needed to have a Systemic lupus erythematosus disease activity index (SLEDAI) score >8, refractory low blood platelets, or severe lupus-related kidney disease (lupus nephritis). As with the first publication, patients received a single intravenous infusion of 1 million mesenchymal stem cells per kilogram of body weight. The net result of the study: There were no serious side effects; SLEDAI scores significantly improved at 1, 3, 12, and 24 months; protein in the urine (secondary to kidney disease) significantly improved at 1, 3, 12, and 24 months; and the percentage of the immune-regulating T-regulatory cells significantly increased at 1 week, 3 months and 6 months after treatment.
In the next article3, Sun used donor umbilical cord-derived mesenchymal stem cells instead of bone marrow-derived. In our laboratory experience umbilical cord MSCs can undergo approximately twice the number of "doublings" as their adult-derived bone marrow counterparts. Since the doublings are logarithmic, the actual number of cells that can be grown from a single umbilical MSC is more than 99 percent more than from adult bone marrow. Simply put, umbilical cord MSCs are more potent than MSCs taken from an adult. The patients in this study were refractory to standard treatment and had life-threatening visceral involvement. The take home from this study was: "Significant reduction in disease activity was achieved in all patients, and there has been no recurrence to date and no treatment-related deaths." As with the previous bone marrow MSCs study there was significant improvement in the SLEDAI scores and kidney function, and a significant increase in the number of T-regulatory cells.
The fourth article4 describes four patients with a severe lung complication from lupus and were treated again with umbilical cord MSCs. Diffuse alveolar hemorrhage, or DAH, literally means that the small grape-like structures of the lungs are bleeding. The condition comes with a 50% mortality rate. All of the patients improved with treatment and all survived. To quote from the authors, "All the four patients showed dramatic improvements of their clinical manifestations." The hemoglobin, which was low because of the bleeding improved in the patients, and was normal 6 months after treatment. Improvement was also seen in the platelet levels of the two patients with dangerously low platelet levels prior to the treatment.
In the Sun groups latest article5 they sum up four years of experience in treating severe and refractory SLE with MSCs. This excerpt is taken from the summary of the article: "During the 4 years follow up and with a mean follow up period of 27 months, the overall rate of survival was 94% (82/87). Complete clinical remission rate was 28% at 1 year (23/83), 31% at 2 years (12/39), 42% at 3 years (5/12) and 50% at 4 years (3/6). Rates of relapse were 12% (10/83) at 1 year, 18% (7/39) at 2 years, 17% (2/12) at 3 years and 17% (1/6) at 4 years. The overall rate of relapse was 23% (20/87). Disease activity declined as revealed by significant changes in SLEDAI score, levels of serum autoantibodies, albumin and complements. A total of 5 patients (6%) died after MSCT from non-treatment-related events in 4 years follow up, and no transplantation-related adverse event was observed. Allogeneic MSCT resulted in the induction of clinical remission and improvement in organ dysfunction in drug-resistant SLE patients. No transplantation-related adverse event was observed."
Dr. Sun and his team continue their work, here is a link to their current study: Umbilical Cord Derived Mesenchymal Stem Cells Transplantation for Active and Refractory Systemic Lupus Erythematosus
Despite the benefits seen in the Nanjing MSC lupus trials, there is only one other MSC clinical trial registered on the NIH website, ClinicalTrials.gov that is currently recruiting patients with lupus.
For more information, visit Neil Riordan's website.
References
- Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009 Jun;27(6):1421-32.
- Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010 Aug;69(8):1423-9.
- Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010 Aug;62(8):2467-75.
- Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 2012 May;31(5):841-6.
- Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years experience. Cell Transplant. 2012 Oct 31. [Epub ahead of print]


